Fourth, the factors affecting the deformation
1.plastic raw materials
Plastic raw material characteristics for the deformation of molded products is a huge impact. Different raw materials have different molecular structure and intermolecular forces, which are expressed as different mobility, orientation characteristics, shrinkage characteristics, and mechanical and physical properties, so the resulting shrinkage rate is not the same.
The different molecular structures of plastic materials determine the type of plastic materials; and its internal additives determine the different properties of the same plastic, including fluidity, anti-degradation properties, flexibility, flame retardancy, and UV resistance and other properties.
Although most of the time, as a mold factory and injection molding plant, can not decide which plastic materials to use, but understand the differences in plastic materials, and its impact on product deformation, for us to analyze the problem, to solve the problem is very helpful.
With this knowledge, we can predict the deformation to a greater extent in the early stage, so that on the one hand we can give reasonable advice to our customers (e.g. justified advice to relax unrealistic shape and size tolerance requirements; advice on reasonable design structure to compensate for deformation), and on the other hand we can design effective preventive measures in the mold (e.g. targeted design of pouring system and cooling system).
There are countless types of plastics, and more importantly, the freedom of plastic formulation is very high, and the production of plastic materials can be customized or modified according to the needs of the user or application, which allows the variety of plastic materials to be extended to nearly unlimited. In general, there are two main categories of plastics used in injection molding production: thermoset plastics and thermoplastics, thermoset plastics are not in the scope of this paper, and therefore not described in detail here.
Thermoplastics are divided into crystalline and non-crystalline plastics according to their tendency to crystallize or not. In practice, it is almost impossible to provide sufficient conditions for crystalline plastics to fully crystallize, and since in most cases crystalline plastics are not fully crystallized, most of the time we call them semi-crystalline plastics.
Crystallization refers to the formation of an orderly and neatly folded arrangement of the plastic molecular chains during the cooling and curing process. The nature of crystallization makes the shrinkage rate of crystalline plastics naturally higher than that of non-crystalline plastics, because the molecular chains are neatly arranged and occupy relatively less space, thus exhibiting a higher shrinkage rate. Just as a diligent person who tidies up the house appears to have a lot of space, the same is true.
But not all plastic materials have a tendency to crystallization, some plastic materials whose molecular chains in the cooling and curing process does not have a tendency to crystallization, but tends to random free winding, molecular chains are tightly wound with each other randomly, the more tightly wound, until completely cured. One of the direct manifestations of crystallization and free winding behavior in plastic material properties is that: crystalline materials shrink more, usually more than 1%; while non-crystalline materials shrink less, usually less than 1%. By this point, we can be based on the shrinkage value of the material roughly separate crystalline materials and non-crystalline materials.
Why some plastic materials have a tendency to crystallize during the cooling and curing process of the molecular chain, and some do not? This is determined by the molecular structure. Relatively speaking, crystalline plastic molecular chain structure is relatively simple, there is no or little complex branch chain, this molecular structure makes it conducive to their own folding, the formation of crystallization. Non-crystalline plastic molecular chain structure is relatively complex, with many branch chains, this complex structure itself is not conducive to its orderly and neat folding, inhibiting its crystallization tendency. See the following figure molecular chain 3D model diagram.
The overall 3D model of PP molecular chain, PP is a crystalline plastic, its molecular chain branching structure is relatively simple, with fewer branching chains, so it can be folded and arranged in an orderly manner during the cooling and curing process, i.e. crystallization.

The overall 3D model of the PC molecular chain is a non-crystalline material, and as you can see from the diagram, its molecular chain structure is very complex, with many branching chains, a structure that is naturally not conducive to crystallization.
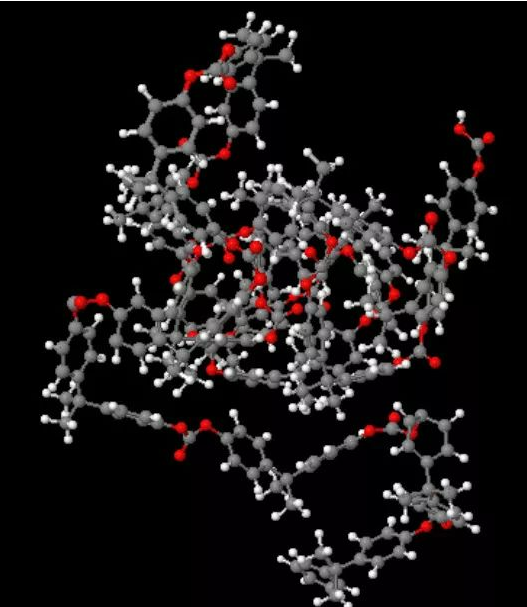
Generally speaking, the more complex the molecular monomer structure of a plastic, the less fluid it is when it forms long chains in the polymerization reaction. This is because the longer the molecular chain, the higher the degree of intertwining, and plastic flow behavior is essentially the behavior of molecular chains moving each other, the longer the molecular chain, the more naturally determined the material is difficult to flow.
Whether it is crystalline plastic or non-crystalline plastic, its molecular form in the molten state is the same, the molecules are naturally curled state, like the earthworm, as can be imagined, the more complex molecular chain structure, the longer the molecular chain, driven by external forces, the difficulty of molecules to move with each other increases, as shown by the viscosity increases, the plastic is difficult to flow.
For semi-crystalline plastics, the degree of crystallization affects the shrinkage of the product. Crystallization requires corresponding conditions, time is a necessary and sufficient condition, and another condition is the content of nucleating agents. High mold temperature provides more sufficient time for molecules to crystallize and therefore the degree of crystallization is higher. The high degree of crystallization leads to an increase in product shrinkage i.e. smaller size.
The increased crystallinity increases the denseness of the product quality and therefore the robustness of the product, but also the brittleness as a result. But on the other hand, the higher the shrinkage, the more difficult it is to control the deformation and dimensions of the product. So when the size and deformation of a product is a key concern, it is not wise to choose a crystalline material such as PP. (But for some mechanical transmission products such as gears and crankshafts, PA or POM materials are needed. These products are designed to be very rigid, so their shrinkage does not cause severe deformation of the product.
And PA and POM materials natural self-lubricating and good mechanical properties to meet the application of these products, although the size requirements are high, but still choose. POM and PA are semi-crystalline materials.) The higher the content of nucleating agents, the more rapidly the plastic can crystallize, so that the degree of crystallization can reach a very high degree in a limited period of time.
Generally speaking, semi-crystalline materials, due to their crystalline nature, determine their molecular structure is not too complex, so generally speaking, crystalline materials are better flow, such as PP, PA, POM, PE, PBT, and so on.
Non-crystalline plastics, on the other hand, generally have a more complex molecular structure than semi-crystalline plastics. Due to the complexity of molecular structure, it is not naturally conducive to crystallization. In the glassy state, the molecular chains of non-crystalline materials are tightly and randomly entangled, and the tightness of the entanglement is determined by the conditions in the production process.
Like the crystallization of semi-crystalline materials, the random winding of molecular chains of non-crystalline materials also requires conditions, time being the necessary and sufficient condition. Therefore, the higher the temperature of the mold, the longer the winding time is provided, and therefore the higher the degree of winding, the greater the shrinkage. The tighter the winding, the better the mechanical properties such as strength, impact resistance, etc.
Because of the complex molecular chain structure of non-crystalline materials, they are relatively less fluid and require higher injection and holding pressures than semi-crystalline materials. The higher the melting temperature of the material means that the temperature difference between it and the glass transition temperature is greater. The material will release more heat when it drops below the glass transition temperature, so it will take longer time, which also provides a longer time for the winding of plastic molecules, and therefore the greater the shrinkage.
The shorter the molecular chain of non-crystalline material, the simpler the structure, which means the better its fluidity, so it is easier to transfer the pressure during the injection molding process, the better the pressure retention and shrinkage, and the shrinkage and deformation are more easily controlled.
It should be noted that the above-mentioned increase in mold temperature and melt temperature can promote the shrinkage of the plastic material and make the shrinkage of the product bigger. But on the other hand, higher mold temperature and melt temperature also provide more favorable conditions for shrinkage.
The crystallization process of semi-crystalline materials and the random winding process of non-crystalline materials is a process of material shrinkage, shrinkage is accompanied by the release of excess space, and the released space is compensated by the melt glue that comes in from the pressurized shrinkage. The higher the mold temperature and the higher the melt temperature, the lower the melt viscosity and the more melt can enter the mold cavity to make up the shrinkage.
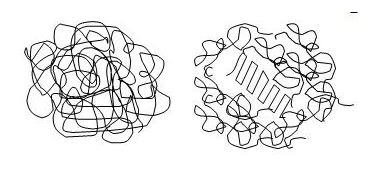
In this case, if the runner gate size is large enough and the holding time is long enough, the product does not necessarily shrink more at high mold temperature and high material temperature; the product has more material in the same volume of cavity, and with a higher degree of molecular crystallization/entanglement, the product has a denser microstructure, which is generally good for the product quality. In this case, it is possible that the shrinkage of the product becomes smaller. The molecular microstructure of thermoplastics in the glassy state is shown in Figure 4.5.
Schematic diagram of microstructure in the glassy state of non-crystalline and semi-crystalline plastics. The left figure shows a non-crystalline plastic with molecular chains in a randomly intertwined state. The right figure shows a semi-crystalline plastic, where the middle part of the neatly arranged part is the crystalline region, and the outer part is wrapped around the non-crystalline region.
The molecular chains of non-crystalline plastics show a random winding pattern, and when heat is absorbed, the intermolecular gap expands and the molecules slowly unwind. Therefore, for non-crystalline plastics, there is only a clear glass transition temperature (Tg), but not a clear melting point.
The melting process is like a piece of cream, which melts gradually as the temperature rises. When the temperature rises above the glass transition temperature (Tg), the molecular chains wrapped around the crystalline region begin to loosen and expand, and their melting behavior is the same as that of non-crystalline plastics. flow.
In the process of lattice melting, although the material absorbs heat, its temperature does not rise significantly, this temperature point is the melting temperature of the material (Tm). This temperature point is the melting temperature of the material (Tm), just as ice melts into water. Thus semi-crystalline plastics usually have a more pronounced melting point.
Due to the presence of crystalline masses in semi-crystalline plastics, the stability of the lattice is high and therefore the stability of the product is also high. In semi-crystalline materials, only their crystalline region is the real curing region, while their non-crystalline region, even after injection molding, under certain suitable conditions, will still appear to be dependent on the crystalline region for recrystallization, and this phenomenon results in post-shrinkage of the product.
In the case of non-crystalline materials, the internal organization is never really cured, and even after injection molding, when the conditions are right, the molecules will continue to wind, which is why non-crystalline plastics are relatively less stable.
The shrinkage characteristics of non-crystalline plastics are isotropic due to the random winding shrinkage pattern of the molecular chains during cooling and curing. By isotropic shrinkage, we mean that the shrinkage rate of the plastic is basically the same in the flow direction and the vertical flow direction. Isotropic shrinkage is relatively uniform, so it is easy to control, and its deformation is relatively easy to control.
Semi-crystalline plastics due to its crystallization, its shrinkage characteristics show a slight anisotropy, that is, the flow direction and vertical flow direction shrinkage rate is not the same. In general, the shrinkage in the flow direction will be slightly greater than the shrinkage in the vertical flow direction, and the higher the degree of crystallization, the greater the difference. This is because of the influence of flow orientation, in flow, the molecular chains are roughly neatly arranged along the flow direction, and when cooling occurs crystallization, the extension direction folds into an orderly arrangement, which leads to a larger shrinkage relative to the vertical dimensional shrinkage, resulting in anisotropy of shrinkage.
In conclusion, the shrinkage of semi-crystalline plastics is more difficult to control than that of non-crystalline plastics, and the physical changes of their molecular structure during cooling and curing are more complicated. Therefore, when designing molds for injection molding semi-crystalline plastics, it is more important to consider the rheological and shrinkage characteristics of the material carefully.
Plastic material additives, including plasticizers, flexibilizers, flame retardants, etc., these additives to improve the processability and usability of plastic will, to a certain extent, change the flow characteristics of plastic and shrinkage characteristics. Usually additives such as plasticizers that improve material flow are beneficial to product deformation control because improved flow means lower pressure required for injection molding, lower internal stress in the product, and therefore improved deformation.
Material fillers, including high aspect ratio fillers such as fiber and low aspect ratio fillers such as talc, also have a significant impact on the shrinkage characteristics of the plastic. Additives with low aspect ratios such as talc have relatively little effect on the shrinkage characteristics of plastics, and only reduce the shrinkage rate of the material without increasing the anisotropy of the shrinkage.
Fillers such as talc and glass beads are isotropic in shape and therefore do not cause anisotropic shrinkage of the material. Because they do not shrink by themselves or shrink much less than the plastic substrate, they simply reduce the overall shrinkage of the material. Since these additives are harder than the plastic base material, their addition increases the strength and modulus of elasticity of the material.
However, the effect of high aspect ratio additives such as fibers on the shrinkage characteristics of plastics is enormous, and can be said to completely change the shrinkage characteristics of plastic, increasing the anisotropy of the material shrinkage, so that it presents a shrinkage characteristics relative to the opposite of unfilled plastic. The reason for this is that fibers and polymers are coupled together, constraining the shrinkage of the polymer.
Since the fibers themselves are non-shrinking and do not tend to twist in their free state, an explanation in terms of the earthworm theory described earlier is that this earthworm has a thin stick tied to it to increase its strength (plus glass fiber material), and its free twisting nature is restricted due to the restraint of the stick, thus exhibiting a diametrically opposed shrinkage property.
Thus it shows a very small shrinkage along the flow direction and a large shrinkage in the vertical flow direction due to the molecular gap, plus the volume shrinkage rate of the material is certain, when the shrinkage is suppressed in one direction, a larger shrinkage will inevitably occur in the other direction to compensate. In contrast, the shrinkage of unfilled material along the flow direction is slightly larger than that in the vertical flow direction due to the nature of molecular chain entanglement.
The length of the molecular chain of the material and the complexity of the molecular chain structure have a great influence on the molding process and therefore affect the effect of the process on deformation. The length of the molecular chain and the structure of the molecular chain of the material also determine the mechanical and mechanical properties of the material, so it will affect the stability of the structure of the product and the influence of the structure of the product on the deformation.
To sum up:
The structure of the molecular chain of a plastic determines the type of plastic, while the addition of various additives and fillers changes the properties of the same material, including physical properties, rheological properties, and resistance to various degradations.
The complexity of the molecular chain of a plastic determines to some extent whether the plastic has a tendency to crystallize or not. Since crystallization is the orderly folding and arranging of plastic molecules during cooling and curing, an overly complex molecular chain structure is not conducive to crystallization. Therefore, semi-crystalline plastics usually have a relatively simple molecular chain structure, while non-crystalline plastics have a relatively complex molecular structure.
The higher the degree of crystallization of a semi-crystalline plastic, the greater its shrinkage. Crystallization requires conditions, and time is the most important one. The longer it takes for the product to cool, the higher the degree of crystallization.
Non-crystalline plastics shrink less than semi-crystalline plastics because of the random winding of the molecular chains during the cooling and curing process. Random winding shrinkage also takes time, and the longer the time, the tighter the winding, the greater the shrinkage.
The shrinkage of semi-crystalline plastics is slightly anisotropic, and the greater the crystallinity, the higher the degree of anisotropy. In contrast, the shrinkage characteristics of non-crystalline plastics are basically isotropic. The material shows anisotropic shrinkage, and its shrinkage behavior is more difficult to control, which means that the deformation of the product is more difficult to control.
The addition of various fillers will change the rheological properties and shrinkage performance of the material. The addition of low aspect ratio fillers such as talc will basically not change the anisotropy of material shrinkage, but the addition of high aspect ratio fillers such as fibers will lead to severe anisotropy of material shrinkage. Therefore, it is more difficult to control the shrinkage and deformation of fiber-added plastics, and the flow shrinkage behavior of glass fiber materials needs to be carefully developed in order to develop a high-quality mold.